
FEATURES
| FEATURES | BENEFITS |
|---|---|
|
Full compression level of around 40 mmHg at the ankle. 1.Comfortable:
2.Consistent:
3.Continuous Pressure:
|
INDICATIONS
- Treatment, in adults, of venous leg ulcer and/or lower limb oedemas where strong compression is recommended.
CONTRAINDICATIONS
- Moderate or severe arterial conditions, notably with recent Ankle Brachial Pressure Index (ABPI) less than 0.8
- Patients suffering from phlegmatia coerulea dolens, septic phlebitis
- Oedema from congestive cardiac failure
- Epifascial arterial bypass
- Known hypersensitivity to any of the component in UrgoK2
PRECAUTIONS
- Single use: do not wash to maintain the performances of the medical device.
- Do not sterilize.
- Do not use in direct contact with a wound.
- In the event of arterial bypass, cardiac failure, peripheral neuropathy (for example in the case of diabetes), infected peri-ulcer dermatitis or acute cellulitis (erysipelas), use the compression system after specialist referral and under close medical monitoring.
- In case of ABPI more than or equal to 1.3, suspicion of media calcific sclerosis or history of revascularization, an extensive vascular assessment is recommended before initiating the compression therapy.
- In case of infected leg ulcer, start treating infection in combination with compression therapy.
- The efficacy of UrgoK2 has been tested and validated for the application of KTech and KPress together.
- Follow the application method given to achieve the recommended therapeutic pressure. The PresSure System has been specially designed to guide the application of the bandages.
- This compression system should be worn continuously day and night until the next care at the discretion of the clinician.
- If experiencing discomfort, pain or skin reactions whilst wearing this product, patient should contact his/her healthcare professional.
- Any serious incident linked to the product must be reported to the manufacturer immediately.
For a complete list of precautions, contraindications, indications, please reach out to your local sales representative and consult the Instructions for Use (IFU)
DIRECTIONS FOR USE
PRODUCT OPTIONS
| Product Size | Units/Box |
|---|---|
| Ankle Size: 18-25cm | 1 kit/ box |
| Ankle Size: 25-32 cm | 1 kit/ box |
CLINICAL EVIDENCE
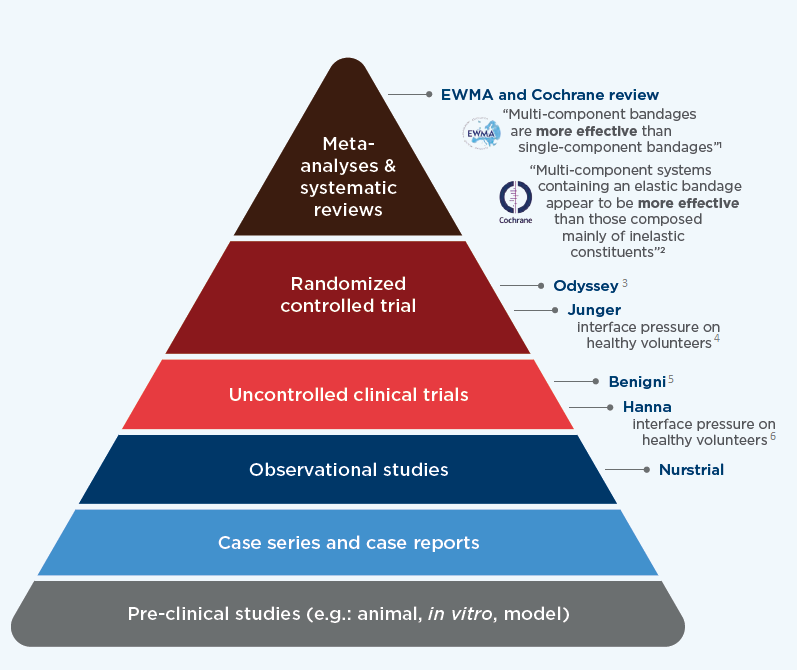
1.European Wound Management Association (EWMA). Position Document. Understanding compression therapy. London: MEP Ltd; 2003:13-14.
2.Meara S, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;14;11:CD000265.
3.Lazareth I et al. Efficacy of two compression systems (UrgoKTwo vs Profore) in the local management of venous leg ulcers: Results of a European randomized controlled trial. J Wound Care 2012;21(11):553-4,556, 558
4.Junger M, et al. Comparison of interface pressures of three compression bandaging systems used on healthy volunteers. J Wound Care. 2009; 18(11):474-80.
5.Benigni JP, Lazareth I, Parpex P, et al. Efficacy, safety and acceptability of a new two-layer bandage system for venous leg ulcers. J Wound Care. 2007;16(9):385-90.
6.Hanna R, Bohbot S, Connolly N. A comparison of interface pressures of three compression bandage systems. Br J Nurs. 2008;17(20):S16-24.

