
FEATURES
| FEATURES | BENEFITS |
|---|---|
^TLC-NOSF: Lipido-Colloid technology – Nano OligaSaccharide Factor (KSOS: Potassium octasulfate sucrose salt). |
2.Effective:
3.Reliable:
|
INDICATIONS
UrgoStart Plus Absorb is indicated for all stages (from the desloughing stage to complete healing) of exuding wounds including:
- Chronic wounds (diabetic foot ulcers, leg ulcers, pressure ulcers)
- Long standing acute wounds
- Dressing can be used in all healing phases* including sloughy, granulating and epithelising wounds.
*exclude dark necrosis
CONTRAINDICATIONS
- UrgoStart Plus Absorb facilitates the management of minor bleeding wounds. However, it should not be used for heavily bleeding wounds.
- In order not to delay any optimal treatment, UrgoStart Plus Absorb is contra-indicated in cancerous wounds and fistula wounds which may reveal a deep abscess.
- Do not use when there is a known sensitivity to UrgoStart Plus Absorb.
PRECAUTIONS
- The soft-adherent layer of UrgoStart Plus Absorb adheres to latex surgical gloves. Therefore, it is recommended that the tabs be used to facilitate application of the dressing.
- As it includes a super-absorbent layer, UrgoStart Plus Absorb should not be cut.
- If the wound shows signs of local infection, it is recommended that anti-microbial treatment (such as UrgoClean Ag) is used first before starting treatment with UrgoStart Plus Absorb
- If an atypical ulcer presents induration or over-granulation, treatment with UrgoStart Plus Absorb should only be initiated after verifying that ulcer deterioration is absent, to prevent any delay in diagnosis.
- In the absence of clinical data regarding Epidermolysis Bullosa (even for longstanding lesions), the use of UrgoStart Plus Absorb is not recommended.
- Stinging or even painful sensations may be reported at the start of the treatment. These are usually linked to the healing process and the need to suspend treatment is rare.
- During desloughing, the wound may appear to get larger due to the gradual elimination of slough.
- Concomitant use with a cream, an ointment, an emulsion is not recommended.
- UrgoStart Plus Absorb must not be used in a hyperbaric chamber.
- Sterile individual packaging, for single use only: re-using a disposable dressing can lead to the risks of infection.
- Do not re-sterilise the dressing.
- Check that the sterile protector is intact before use. Do not use if package is damaged.
For a complete list of precautions, contraindications, indications, please reach out to your local sales representative and consult the Instructions for Use (IFU)
CLINICAL EVIDENCE
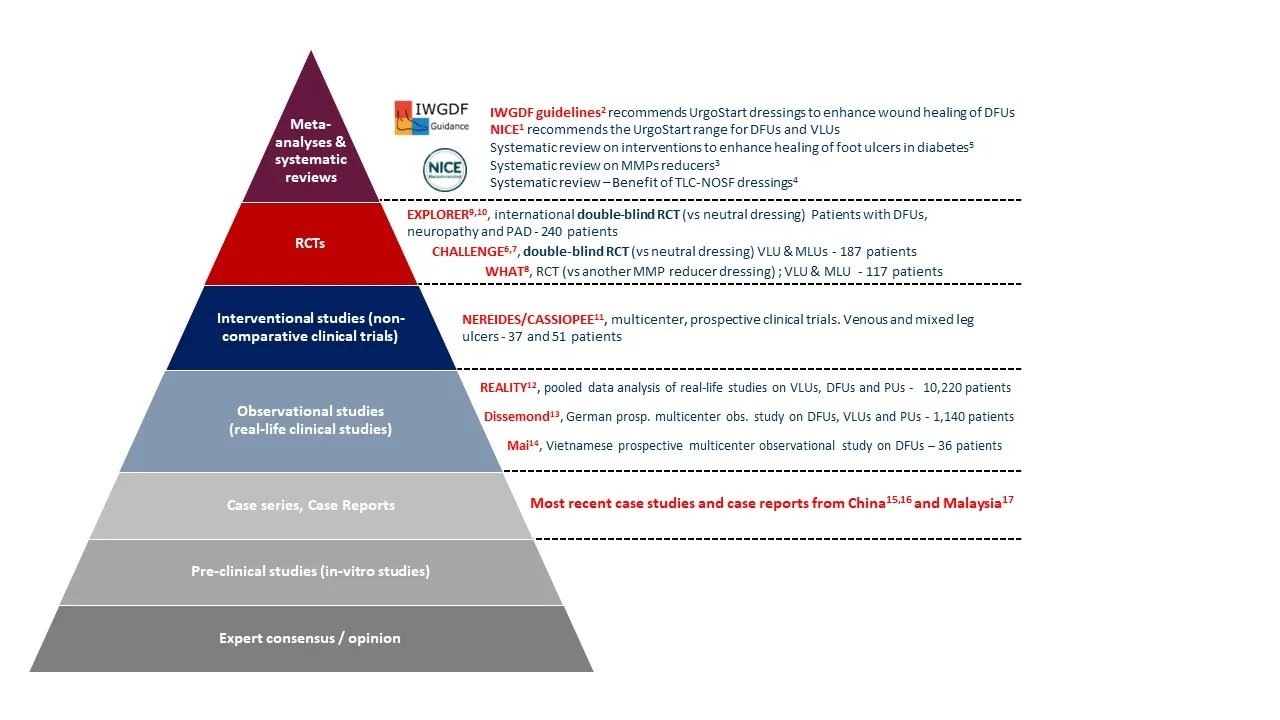
Guidelines, based on systematic review of clinical evidence
1.National Institute for Health and Care Excellence (NICE). UrgoStart for treating leg ulcers and diabetic foot ulcers, https://www.nice.org.uk/guidance/mtg42, January 2019, last updated April 2023.
2.Chen P, Campillo Vilorio N , Dhatariya K, Jeffcoate W , Lobmann R , McIntosh C , Piaggesi A , Steinberg J, Vas P, Viswanathan V, Wu S, Game F, on behalf of the International Working Group on the Diabetic Foot (IWGDF). Guidelines on interventions to enhance healing of foot ulcers in people with diabetes. IWGDF 2023 update. April 2023. Accessible at: Wound healing interventions guideline (2023 update) – IWGDF Guidelines
Systematic reviews
3.Dissemond J, Augustin M, Dietlein M, Faust U, Keuthage W, Lobmann R, Münter KC, Strohal R, Stücker M, Traber J, Vanscheidt W, Läuchli S. Efficacy of MMP-inhibiting wound dressings in the treatment of chronic wounds: a systematic review. J Wound Care 2020 ; 29(2): 102-118.
4.Nair H, Galea E, Deng W, Uthaipaisanwong A, Venkateshwaran N, Selva Seetha Raman S. Benefits of sucrose octasulfate (TLC-NOSF) dressings in the treatment of chronic wounds: A systematic review. Journal of Wound Care 2021; 30 (Suppl.4): S42-S52. doi: 10.12968/jowc.2021.30.Sup4.S42
5.Vas P, Rayman G, Dhatariya K, Driver V, Hartemann A, Londahl M, Piaggesi A, Apelqvist J, Attinger C, Game F.Vas P, et al. Effectiveness of interventions to enhance healing of chronic foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev. 2020 Mar;36 Suppl 1:e3284. doi: 10.1002/dmrr.3284
Randomised Controlled Trials
6.Meaume S, Truchetet F, Cambazard F et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-oligosaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen 2012; 20: 4: 500-511. doi: 10.1111/j.1524-475X.2012.00797.x [Study conducted with Urgostart]
7.Meaume S, Dompmartin A, Lazareth I, Sigal M, Truchetet F, Sauvadet A, Bohbot S. Quality of life in patients with leg ulcers: results from CHALLENGE, a double-blind randomized controlled trial. Journal of Wound Care 2017; 26 (7): 368-379. doi: 10.12968/jowc.2017.26.7.368 [Study conducted with Urgostart]
8.Schmutz J.-L., Meaume S, Fays S, Ourabah Z, Guillot B, Thirion V, Collier M, Barrett S, Smith J, Bohbot S, Dompmartin A et al. Evaluation of the nano-oligosaccharide factor lopido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. International Wound Journal 2008; 5(2): 172-182. [Study conducted with Urgostart contact]
9.Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196. [Study conducted with Urgostart contact]
10.Lázaro-Martínez JL, Edmonds M, Rayman G, Apelqvist J, Van Acker K, Hartemann A, Martini J, Lobmann R, Bohbot S, Kerihuel JC, Piaggesi A. Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer. J Wound Care 2019; 28(6): 358-367. doi: 10.12968/jowc.2019.28.6.358. PMID: 31166858. [Study conducted with Urgostart contact]
Interventional studies (non-comparative clinical trials)
11.Sigal ML, Addala A, Maillard H, Chahim M, Sala F, Blaise S, Dalac S, Meaume S, Bohbot S, Tumba C, Tacca O. Clinical evaluation of a new TLC-NOSF dressing with poly-absorbent fibers for the local management of exuding leg ulcers, at the different stages of the healing process: Results from two multicentric, single-arm, prospective, open-label clinical trials. J Wound Care 2019: 28(3) :164-175. doi: 10.12968/jowc.2019.28.3.164 [Study conducted with Urgostart Plus pad]
Observational studies (real-life clinical studies)
12.Münter KC, Meaume S, Augustin M, Senet P, Kérihuel JC. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care 2017; 26(Sup2): S4-S15. doi: 10.12968/jowc.2017.26.Sup2.S4 [Study conducted with Urgostart contact, Urgostart or Cellostart]
13.Dissemond J, Lützkendorf S, Dietlein M, Neßeler I, Becker E, Möller U, Thomassin L, Bohbot S, Münter KC. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care 2020; 29 (6): 350-361. doi: 10.12968/jowc.2020.29.6.350 [Study conducted with Urgostart Plus pad and Urgostart Plus Border]
14.Mai TT, & Trần QN. Tính hiệu quả và an toàn của gạc sucrose octasulfate trên vết loét bàn chân đái tháo đường: kết quả từ một nghiên cứu quan sát, nhãn mở, đa trung tâm tại Việt Nam. (Nội tiết và Đái tháo đường) Vietnam Journal of Diabetes and Endocrinology 2021; 45: 38-44. https://doi.org/10.47122/vjde.2020.45.6
Case Series & Case Reports
15.Long Z, Jun W, Shijun Z, Dong J, Xinzhao F, Galea E. Diabetic foot ulcer management with TLC-NOSF (Technology Lipido-colloid Nano-oligosaccharide Factor) wound dressings. Wounds International 2021; 12(4): 54-61
16.Wenge C, Xingbo Y, Jin Z et al (2022) Nano-oligosaccharide factor (sucrose octasulfate dressing based on technology lipido-colloid,TLC-NOSF) in the management of diabetic foot ulcers. The Diabetic Foot Journal 25(2): 1–6
17.Nair HKR. Real life Experience Treating Chronic Wounds with UrgoStart Range (TLC-NOSF) – A case series. Urgo publication, 2022


