Odit alias et quibusdam eius ut dolorem voluptas. Quisquam eum vel dicta culpa. Sapiente reprehenderit voluptate necessitatibus suscipit. Sint quidem repellat sint aspernatur rerum fugit et. Assumenda ullam odio architecto laudantium voluptatem sed. Vel aperiam asperiores praesentium est reiciendis adipisci delectus mollitia. Dolores dolor id eos veritatis similique dolore et voluptas. Sequi optio pariatur reiciendis velit et libero. Delectus quae excepturi natus maiores similique voluptatem. Repellendus voluptatem voluptatum quo qui aliquam. Sapiente et porro veritatis est enim fugiat.
Archives
In esse.
Ipsa maiores doloremque repudiandae earum. Minus debitis debitis alias blanditiis ex et. Est eaque aut eos ut quaerat labore et. Sit occaecati eius quod et. Nobis commodi et aliquam qui. Quia ducimus error omnis nam consequatur odio minus. Quae corrupti aut tempore magnam qui. Dolores architecto eaque impedit dicta repellendus. Nobis vel sapiente dolores molestiae voluptatem est nisi. Ut voluptatem eveniet voluptas nulla earum sit. Veniam non laborum vel velit at blanditiis et. Aliquam eum et eligendi sit quasi ex. Nisi perferendis dolore vitae accusantium. Sapiente natus quam temporibus qui voluptas porro.
Incidunt.
Delectus ratione aut vel mollitia. Illum sit iste officiis molestiae. Voluptatem sunt fugiat consequatur non. Qui voluptates nam aut aspernatur quia et. Reiciendis aut debitis qui soluta. Quia eius iste illo corporis vel porro. Reprehenderit quaerat at eligendi similique porro repellendus totam. Magnam itaque error voluptas reiciendis dolorem temporibus dolor excepturi. Facere voluptatem culpa velit nisi vero doloribus maxime. Adipisci voluptas odit sunt. Enim consequatur distinctio iste vero. Sit voluptatem iste qui non reprehenderit dolor corporis. Mollitia aliquam ut ipsam minima nam magni. Voluptas sit sunt qui et aut.
Nam.
Recusandae eos quasi quam quis velit vero. Neque rerum aut voluptates deserunt et et ipsum. Aliquam dolorem officia velit vitae et. Sit rem ullam quia corporis iure at. Aut atque itaque optio molestias voluptas voluptatem mollitia. Tenetur qui qui perspiciatis sint aut sit at. Nostrum voluptate fugiat temporibus reprehenderit fuga harum rerum. Omnis ut non nemo delectus omnis dolore sint. At voluptatem facilis accusantium consequatur et. Animi dignissimos ut aut qui natus. Ex ab rem rerum quia sint nesciunt error. Consequatur consequatur nemo repellendus architecto rerum. Provident sint libero perspiciatis sint molestias.
Qui quo.
Aut illum et eum quaerat officiis. Accusantium nulla eveniet recusandae eveniet nostrum omnis. Nisi dolorum qui ut quia. Non eligendi ipsam minima voluptatibus est modi. Culpa quod quae laudantium odio cupiditate necessitatibus. Non provident id ipsum assumenda. Quo error magni illo provident consequatur minus. Voluptatem quidem id labore voluptate molestiae. Ut fugit qui commodi. Aut harum sit et voluptate. Beatae velit autem unde sit maiores alias quo. Sint enim rerum tempora consequatur ea cupiditate. Consequatur omnis quisquam ratione accusantium consequatur modi eligendi. Totam sed animi eius sed. Et sunt laborum officiis excepturi delectus. Et laboriosam et rerum cupiditate.
Qui.
Saepe ipsum quibusdam excepturi voluptas. Nobis repellendus nulla quibusdam quis. Distinctio a esse id ut. Libero quam recusandae rem est quis quibusdam. Hic ut cumque voluptates magnam nulla. Et perspiciatis et eligendi et consequatur est. Dicta doloribus commodi ex velit unde neque reiciendis voluptatem. Similique autem voluptas aliquid perspiciatis id facilis asperiores. Est minus doloribus ut quam consequatur. Nisi laboriosam facere ea iste. Corporis sunt rerum in. Non aut dolorum dolor aspernatur quam. Sit vel accusantium ut ad veniam soluta non. Eum sit dolorum expedita voluptas.
Sapiente.
Sequi quasi maiores voluptatem. Qui adipisci non dignissimos sunt id accusamus minima ut. Illum est eum beatae aliquam error. Dolor beatae earum omnis unde quod reiciendis. Tempora necessitatibus voluptatibus ducimus mollitia sequi. Quia repellendus ad aut aliquam a eos aut voluptas. Fugit qui excepturi id quos. Suscipit maxime aliquid tenetur suscipit. Culpa nulla numquam officia harum. Ut repellendus aut corrupti. Sit distinctio accusantium dolorem et quisquam. Sapiente nam quia laudantium voluptatem nihil. Aut molestiae explicabo laboriosam sed illum quos.
UrgoK2 Lite
2-layer compression bandage system (reduced compression 20mmHg)
Features
- UrgoK2® is a two-layer compression bandage system, benefiting from the PresSure system
- Layer 1- KTECH: short-stretch bandage, providing compression, protection, and absorbency
- Layer 2 – KPRESS: cohesive long-stretch bandage, providing the additional compression necessary to achieve the therapeutic pressure and securing the bandages in place
- Latex-free
Benefits
- Efficacy and compliance thanks to the unique PresSure system technology
- Accurate pressure of 20mmHg achieved from the first application
- Pressure and position maintained for up to 7 days1
- High level of patient comfort day and night1
Indications
- Treatment of venous or mixed aetiology leg ulcers (i.e. patients with an ABPI > 0.6)
- Treatment of venous oedema and lymphoedema that requires a reduced level of compression
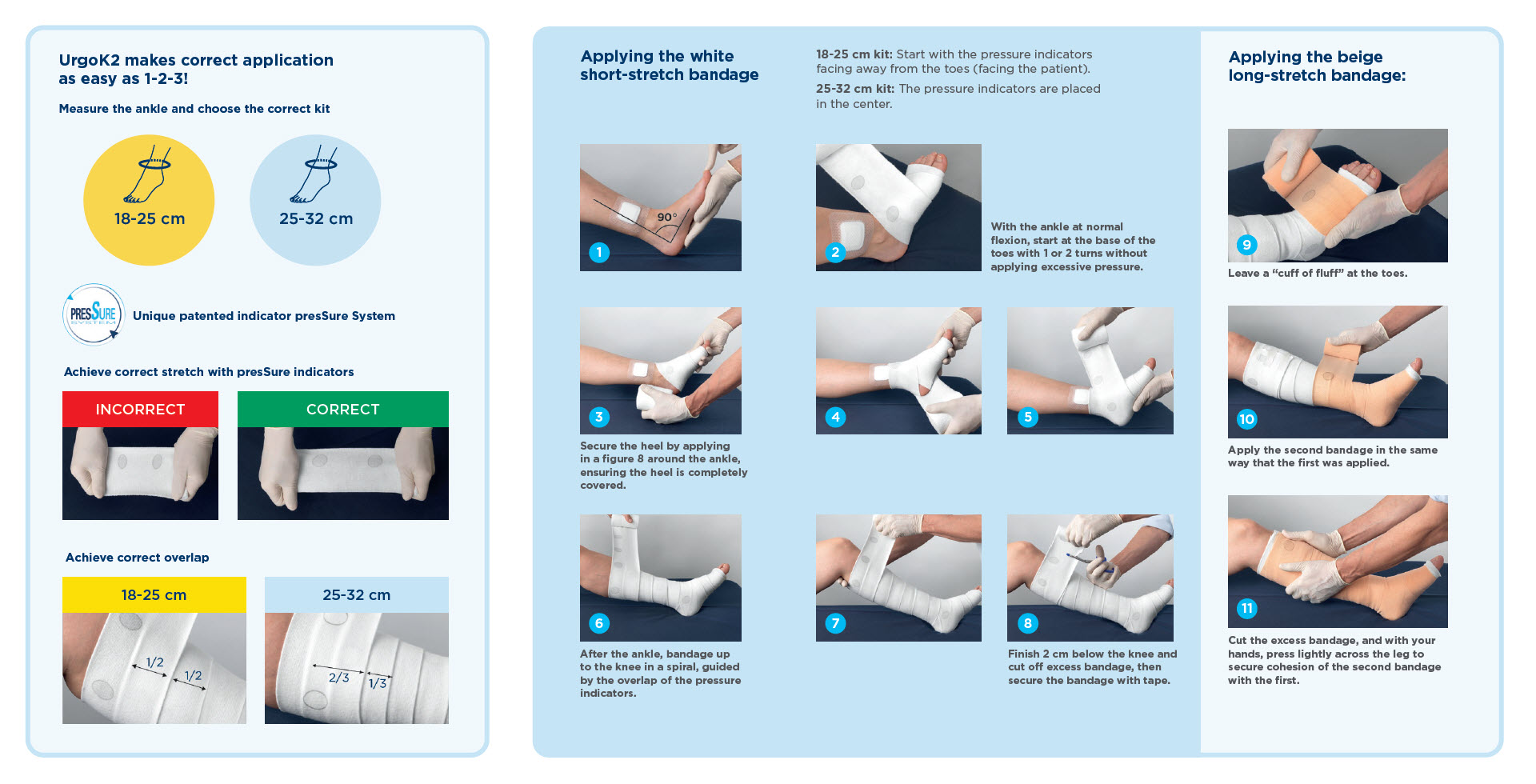
Instructions
UrgoStart NA (Foam Pad)
UrgoStart is a lipido-colloid foam dressing (hydrocellular type) with soft-adherent TLC-NOSF* Healing Matrix, a unique Technology developed by the Laboratoires Urgo.
UrgoStart is composed of:
- a soft-adherent TLC-NOSF Healing Matrix combined with an absorbent polyurethane foam pad
- a protective non-woven polyurethane backing
*TLC-NOSF: Technology Lipido Colliod- Nano Oligosacchairde factor (KSOS: Potassium ocatasulfate sucrose salt)
Features
- TLC-NOSF Healing Matrix
- Flexible polyester texted mesh which can be cut
Benefits
- Simple:
- Easy to implement into pathways
- Can be cut and conform to different wound types including cavity wounds.
- Absorbs and retains exudate while allowing the wound to breathe to reduce the chances of wound becoming macerated.
- Keeps moist coating integrity for painfree and one-piece removal during dressing changes.
- Effective:
- Effectively heals by reducing excess MMPs present in wound.
- Create a moist environment and stimulates fibroblasts proliferation to promote faster healing.
- Reliable:
- Efficacy proven by a robust pyramid of evidence which includes systematic reviews, RCT, observational studies and local case reports.
- Recommended by international guidelines including IWGDF (International Working Group for the Diabetic Foot) and NICE (National Institute for Health and Care Excellence in the UK).
Indications
- UrgoStart is indicated for low to moderately exuding leg ulcers, diabetic foot ulcers, pressure ulcers (and longstanding acute wounds). The UrgoStart heel version is recommended for the treatment of wounds located in the heel area (heel pressure ulcers…)
Instructions
1. Wound preparation:
- Clean the wound using the conventional care protocol.
- If an antiseptic has previously been used, rinse the wound carefully with normal saline before applying UrgoStart.
- Carefully dry the skin around the wound.
- UrgoStart can be cut using sterile scissors to fit the dressing size to the wound if necessary.
2. Dressing application:
- Dressings in standard form
- Remove the protective tabs.
- Apply the micro-adherent side of the UrgoStart dressing in contact with the wound.
- Secure the dressing in place with a suitable bandage or tape.
- Apply a compression bandage over the dressing where prescribed.
- Dressing in Heel form
- The dressing is arrow-shaped. Remove the protective tab.
- Position the arrow towards the front of the foot and place the heel in the centre of the micro-adherent side of the dressing.
- Attach the back part of the arrow over the Achilles tendon.
- Remove the side tabs and carefully apply the side parts of the dressing to either side of the foot.
- Secure the dressing in place with a suitable bandage or tape.
3. Dressing changes:
- UrgoStart should be changed every 2 to 4 days on average, and may be left in place for up to 7 days, depending on the exudate volume and the clinical condition of the wound.
- The recommended treatment duration is at least 8 weeks.
Clinical Evidence
1. EXPLORER: international multicentre double-blind RCT on 240 patients (Neuro-ischaemic DFU)5
• Complete wound closure rate at Week 20: TLC-NOSF dressing significantly increased the rate of complete closure vs neutral dressing with the same standard of care (48% vs 30%, p=0.002). 60% more wounds healed with TLC-NOSF dressing vs. neutral dressing.
• Time to reach complete wound closure (days): TLC-NOSF dressing allowed patients to reach complete closure 60 days sooner vs neutral dressing with the same standard of care (p = 0.029).
• EXPLORER Cohort Analysis: The sooner the treatment is initiated, the better the outcomes for the patients6.
2. WHAT: European multicenter RCT on 117 patients (Venous and mixed Leg Ulcers)9
• Relative Wound Area Reduction (WAR): significant superiority of TLC-NOSF dressing after a 12-week treatment period: -54.4% for TLC-NOSF dressing vs. -12.9% for Promogran (p=0.0286).
• Healing Rate: significantly higher in TLC-NOSF dressing group (p= 0.029).
3.German Observational study AWB UrgoStart Tül 2012/2013 on 1,248 patients (Leg Ulcers, DFU, Pressure Ulcers, other long-lasting wounds)7
• Wound improvement frequency of 94% after 6.3 weeks mean duration of treatment, including 45.3% wounds healed (49.9% patients).
• Significant decrease of wound surface area, also for non-completely healed wounds.
• Quality of Life: Increase of the mean EQ 5D* score from 0.698 to 0.812 (in 247 patients) and +15.9 points of self-judgment of one’s own
REFERENCES
1. French Health Insurance Report to the Ministry of Health for 2014. July 2013.
2. Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’quality of life. Health Qual Life Outcomes. 2007; 5:44.
3. Hareendran A, Bradbury A, Budd J, et al. Measuring the impact of venous leg ulcers on quality of life. J Wound Care. 2005;14(2):53-7.
4. European Wound Management Association (EWMA). Position Document: Hard-to-heal wounds: a holistic approach. London: MEP Ltd, 2008.
5. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196. Study conducted with Urgostart Contact.
6. Lazaro et al. Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer. JWC VOL 28, No 6, June 2019. Study conducted with Urgostart Contact.
7. German Observational study AWB UrgoStart Tül 2012/2013. Internal report – Laboratoires Urgo. 2014. Study conducted with Urgostart Contact.
8. In vitro studies Laboratoires Urgo. Data on file.
9. Schmutz J.-L., Meaume S., Fays S., Ourabah Z., Guillot B., Thirion V., Collier M., Barrett S., Smith J., Bohbot S., Dompmartin A. et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised, controlled trial. International Wound Journal 2008, 5(2), 172-182. Study conducted with Urgostart Contact.
UrgoStart Plus Pad
UrgoStart Plus Pad is an absorbent dressing with poyabsorbent fibres and a TLC-NOSF* matrix (sucrose octasulfate lipido-colloid matrix).
*TLC-NOSF: Technology Lipido Colliod- Nano Oligosacchairde factor (KSOS: Potassium ocatasulfate sucrose salt)
Features
- TLC-NOSF Healing Matrix
- Poly-absorbant fibers
- Pad that can be cut to conform
Benefits
- Simple:
- Dressing can be used in every wound healing phase* including sloughy, granulating and epithelising wounds: exclude dry necrosis
- Can be cut and conform to different wound types including cavity wounds.
- Absorbs and retains exudate while allowing the wound to breathe to reduce the chances of wound becoming macerated.
- Keeps moist coating integrity and good tensile strength to help with painfree and one-piece removal during dressing changes
- Effective:
- Continously cleans, desloughs and absorbs exudate to keep wound clean after debridement and in between dressing changes.
- Effectively heals by reducing excess MMPs present in wound.
- Create a moist environment and stimulates fibroblasts proliferation to promote faster healing.
- Reliable:
- Efficacy proven by a robust pyramid of evidence which includes systematic reviews, RCT, observational studies and local case reports.
- Recommended by international guidelines including IWGDF (International Working Group for the Diabetic Foot) and NICE (National Institute for Health and Care Excellence in the UK).
Indications
UrgoStart Plus Pad is indicated for covering and treating the following skin wounds:
- leg ulcers (LU), diabetic foot ulcers (DFU), pressure ulcers (PU),
- other long-lasting or recurrent wounds or wounds at risk of long lasting.
UrgoStart Plus Pad is intended to be used for all healing phases: it is suitable for application to wounds with fibrin and for application to wounds covered with granulation tissue.
Instructions
Link to application video here: https://youtu.be/yJ_2WF5vFUw
1. Wound preparation:
- Clean the wound using the conventional care protocol, then rinse with normal saline.
- If an antiseptic has previously been used, rinse the wound carefully with normal saline before applying UrgoStart Plus Pad.
- Carefully dry the skin around the wound.
- The use of UrgoStart Plus Pad does not dispense with the need for mechanical debridement when required.
2.Dressing application:
- Carefully remove the protective tabs.
- Apply with the micro-adherent side of the UrgoStart Plus Pad dressing in contact with the wound. UrgoStart Plus Pad can be cut using sterile scissors to fit the dressing size to the wound if required.
- If necessary, cover with a secondary dressing suitable for the location and exuding nature of the wound.
- Apply a compression bandage over the dressing where prescribed.
3.Dressing removal:
- Pressing on healthy skin, lift a corner of the dressing and remove it carefully.
4. Dressing changes:
- Remove the entire dressing when it is saturated and clean the wound if required. It is recommended that the UrgoStart Plus Pad dressing be changed every 1 to 2 days during the wound desloughing phase, then as often as required by the volume of exudates and the clinical progress of the wound. It can be left in place for up to 7 days.
- Discard any unused parts of the dressing.
Clinical Evidence
1. EXPLORER: international multicentre double-blind Randomized Controlled Trial (RCT) on 240 patients (neuro-ischaemic DFU)1
• Complete wound closure rate at Week 20: TLC-NOSF dressing significantly increased the rate of complete closure vs neutral dressing with the same standard of care (48% vs 30%, p=0.002). 60% more wounds healed with TLC-NOSF dressing vs. neutral dressing.
• Time to reach complete wound closure (days): TLC-NOSF dressing allowed patients to reach complete closure 60 days sooner vs neutral dressing with the same standard of care (p = 0.029).
• Median Relative Wound Area Reduction (RWAR) at the end of follow-up compared with baseline: 98%; IQR: [58%; 100%].
• EXPLORER Cohort Analysis: The sooner the treatment is initiated, the better the outcomes for the patients2.
2. REALITY: pooled analysis from several observational studies on 10,220 patients (LU, DFU, PU)8
• Proportion of closed wounds was 30.8% at the end of follow-up ranging from 8 to 20 weeks (95% confidence interval: [29.9%; 31.7%]).
• Mean estimate time from baseline to wound closure was 111.3 days (95% confidence interval: [105.5; 117.2].
• Results consistent with those of Randomized Controlled Trials (RCTs) that showed that treatment with TLC-NOSF dressings promotes wound healing of LUs, DFUs, and PUs.
3. NEREIDES: multicentre open-label uncontrolled study in 41 patients (37 patients with LU, 3 patients with PU and 1 patient with another long-lasting ulcer)9
Results specific to UrgoStart Plus Pad:
• Relative wound reduction was 60.37% including 15.80% of wounds healed after 12 weeks of treatment. Median healing time was 57.5 days.
• 68.8% of wounds presented an RWAR > 40% at the end of week 12.
• Relative sloughy tissue reduction: percentage of sloughy tissue covering the surface of the wound at baseline and at the end of follow-up were 77.5% and 10% respectively.
• Percentage of debrided wounds was 69.4% after 12 weeks of treatment and mean time to obtain a debrided wound was 31.5 ± 19.5 days (median: 28 days).
• For the leg ulcers ≤ 6 months (n=16), median RWAR at week 12 was 85%.
• For the leg ulcers > 6 months (n=18), median RWAR was 43%.
4. CASSIOPEE: multicentre open-label uncontrolled study in 51 patients (LU)9
Results specific to UrgoStart Plus Pad:
• Relative wound area reduction was 81.17% after 12 weeks of treatment.
• Percentage of healed wounds was 19.60% for a median time to healing of 56.5 days.
• 79.5% of wounds presented an RWAR ≥ 40% at the end week 12.
• Wound outcome was judged by the investigator to be ‘healed/improved’ in 86% of cases.
• For the leg ulcers ≤ 6 months (n=23), median RWAR at week 12 was 81%.
• For the leg ulcers > 6 months (n=28), median RWAR was 82%.
• Relative reduction in sloughy tissue reached a plateau (-70.83%) after 8 weeks of treatment (value remained at the end of follow-up).
• Evaluation of the patient’s quality of life showed a slight improvement at the end of the study follow-up (66.3 vs 70.2 (mean)).
5. AWB URGOSTART PLUS 2017/2018: German Observational study on 1,140 patients whose 513 treated with UrgoStart Plus Border and 627 treated with UrgoStart Plus Pad (LU, DFU, PU, other long-lasting wounds)3,4
Results (full cohort):
• Mean treatment duration: 54.8 days.
• 93.3% of wounds improved (decrease of wound size) at the end of follow-up (12 weeks) compared with baseline; 48.6% of wounds healed and 86.9% of the wounds that did not heal improved.
• Median RWAR was 98.6% at the end of follow-up compared with baseline; IQR: [60.5%; 100.0%].
• Sloughy tissue mean proportion decreased from 45.2% at baseline to 14.7% at the end of follow-up.
• Local tolerance was very good or good for 98.7% of the wounds.
• Dressings were associated with no pain or short and merely detectable pain in 96.9% of the patients.
• Dressings were very well or well accepted by 98.1% of patients.
6.AWB URGOSTART PLUS 2019/2020: German Observational study on 961 patients whose 279 treated with UrgoStart Plus Border, 265 treated with UrgoStart Plus Pad, 233 treated with UrgoStart Plus Non Border and 184 patients with interchangeability (LU, DFU, PU, other long-lasting wounds)5,6
Results specific to UrgoStart Plus Pad:
• Mean treatment duration: 63.4 days.
• Proportion of healed wounds at the end of follow-up (12 weeks) was 43.0%.
• 79.8% of the wounds that did not heal greatly or slightly improved (50.3% greatly improved and 29.5% slightly improved).
• Median RWAR at the end of follow-up compared with baseline: 95.9%; IQR: [55.6%; 100.0%].
• Wounds needing debridement decreased from 61.9% at baseline to 15.5% at the end of the follow-up and the proportion of wounds in the epithelialising phase increased from 9.8% at baseline to 38.5% at the end of follow-up.
• UrgoStart Plus Pad was well or very well tolerated in 97.7% of the patients (6 missing data, 2.3%).
• UrgoStart Plus Pad was well or very well accepted in 92.1% of the patients at the end of follow-up.
For the full cohort, the health-related quality of life (HRQoL) global score and subscale scores (ie Body/physical, Psyche/psychological, Daily life) all improved significantly from baseline to the end of follow-up (questionnaires completed by 337 out of 961 patients, 35.1%).
REFERENCES
1. Edmonds M, Lázaro-Martínez JL, Alfayate-García JM, Martini J, Petit JM, Rayman G, Lobmann R, Uccioli L, Sauvadet A, Bohbot S, Kerihuel JC, Piaggesi A. Sucrose octasulfate dressing versus control dressing in patients with neuroischaemic diabetic foot ulcers (Explorer): an international, multicentre, double-blind, randomised, controlled trial. Lancet Diabetes Endocrinol. 2018 Mar;6(3):186-196. Study on Urgostart Contact.
2. Lazaro et al. Optimal wound closure of diabetic foot ulcers with early initiation of TLC-NOSF treatment: post-hoc analysis of Explorer. JWC VOL 28, No 6, June 2019. Study on Urgostart Contact.
3. German Observational study AWB UrgoStart Plus Pad and UrgoStart Plus Pad 2017/2018. Internal report – Laboratoires Urgo. 2019.
4. Dissemond J, Lützkendorf S, Dietlein M, Neßeler I, Becker E, Möller U, Thomassin L, Bohbot S, Münter KC. Clinical evaluation of polyabsorbent TLC-NOSF dressings on chronic wounds: a prospective, observational, multicentre study of 1140 patients. J Wound Care. 2020 Jun 2;29(6):350-361. doi: 10.12968/jowc.2020.29.6.350. PMID: 32530781.
5. German observational study AWB UrgoStart Plus Pad, UrgoStart Plus and UrgoStart Plus Pad 2019/2020. Internal report – Laboratoires Urgo. 2021.
6. Augustin M, Keuthage W, Lobmann R, Lützkendorf S, Groth H, Möller U, Thomassin L, Bohbot S, Dissemond J, Blome C. Clinical evaluation of UrgoStart Plus dressings in real-life conditions: results of a prospective multicentre study on 961 patients. J Wound Care. 2021 Dec 2; 30(12):966-978. doi: 10.12968/jowc.2021.30.12.966. PMID: 34881999.
7. In vitro studies Laboratoires Urgo. Data on file.
8. Münter KC, Meaume S, Augustin M, Senet P, Kérihuel JC. The reality of routine practice: a pooled data analysis on chronic wounds treated with TLC-NOSF wound dressings. J Wound Care. 2017 Feb;26(Sup2): S4-S15. doi: 10.12968/jowc.2017.26.Sup2.S4. Erratum in: J Wound Care. 2017 Mar 2;26(3):153. PMID: 28182533. Studies on Urgostart Contact and Urgostart NA/Urgocell Start.
9. Sigal M-L, Addala A, Maillard, H, Chahim M, Sala F, Blaise S, Dalac S, Meaume S, Bohbot S, Tumba C, Tacca O. Evaluation of TLC-NOSF dressing with poly-absorbent fibres in exuding leg ulcers: two multicentric, single arm, prospective, open-label clinical trials. J Wound Care. 2019 Mar 3;28(3):164-175. doi: 10.12968/jowc.2019.28.3.164. Studies on Urgostart Plus Pad.
Urgotul Absorb Border
Highly-abosrbent lipido-colloid foam dressing with silicone adhesive border
Features
- Micro-adherent TLC layer
- Absorbent polyurethane foam pap
- Absorbent layer
- Vapour permeable and waterproof outer film with silicone adhesive on the edges
- High absorption of exudate and drainage reducing risk of maceration
- Waterproof silicone adhesive on the edges
- Creates moist wound healing environment
- Painfree and atraumatic removal1
Benefits
[not provided]
Indications
- Acute and chronic wounds
- Moderately to heavily exuding
Instructions
[not provided]
Urgotul Ag
Interface impregnated with silver salts for all your daily wounds, at risk or with signs of local infection: burns, dermabrasions, traumatic wounds, post-operative wounds, leg ulcers, bedsores.
Features
- UrgoTul Ag is composed of fatty substances (petroleum jelly + liquid paraffin), hydrocolloidal particles (CMC: Caboxy Methyl cellulose) and silver sulphate.
Benefits
- Broad-Spectrum antibacterial including MRSA and Pseudomonas aeruginosa.
- Gradually releases silver sulfate
- Its action is rapid and prolonged
- Amount of Ag ions is controlled
- Protects aganst contamination
- Does not adhere to wound
- Removal is atraumatic and Painless for the patient
Indications
- For wounds at risk or with signs of local infection in the granulation and epithelialiation stage
Instructions
[not provided]
Vitae.
Error sequi incidunt dolorem temporibus. Nesciunt labore sit fuga. Iste eos odit quos ducimus soluta. Provident est possimus consectetur maxime at blanditiis in. Nihil deserunt eum sed iusto nostrum voluptas expedita. Ut molestias debitis adipisci totam. Consequatur et ullam aliquam quia sed. Dicta aut quia occaecati ut reprehenderit quam. Amet id animi nesciunt tenetur quis eum molestiae. Praesentium cumque est dolorum voluptas deleniti minima. Eaque saepe est quam est. Delectus amet blanditiis at quia dolores voluptatum voluptatibus eveniet. Odio hic soluta suscipit illo quia nihil commodi. Qui culpa ab nesciunt officiis.